- José A. Hernández Cortés, Group of Fruit Biotechnology, CEBAS-CSIC (Murcia, Spain)
A recent work, carried out in our laboratory, studied the role of polyamines in the salt stress adaptation in grapevine (Vitis vinifera L.) plantlets.
Salinity is one of the most important stress factors which limits the growth and development of plants by altering their morphological, physiological and biochemical attributes. Salinity induced a water deficit as well as an ionic toxicity in the plants resulting in an alteration in the ionic homeostasis. In addition to the osmotic and toxic effects, salt stress is also manifested as an oxidative stress, contributing all these factors to the deleterious effects of salinity in plants (Hernández et al., 2001; 2003). To mitigate and repair damage initiated by ROS, plants have developed a complex antioxidant defense system. The primary components of this system include carotenoids, ascorbate, glutathione, tocopherols and enzymes such as superoxide dismutase (SOD, EC 1.15.1.1), catalase (EC 1.11.1.6), glutathione peroxidase (GPX, EC 1.11.1.9), peroxidases and the enzymes involved in the ascorbate-glutathione cycle (ASC-GSH cycle; Foyer and Halliwell 1976): ascorbate peroxidase (APX, EC 1.11.1.1), dehydroascorbate reductase (DHAR, EC 1.8.5.1), monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) and glutathione reductase (GR, EC 1.6.4.2) (Noctor and Foyer 1998).
Plant polyamines (PAs) have been suggested to play important roles in morphogenesis, growth, embryogenesis, organ development, leaf senescence, and abiotic and biotic stress responses (Kusano et al., 2008). Therefore, homeostasis of cellular PA levels is also a defensive strategy that plants have developed to cope with adverse situations (Chinnusamy et al., 2005; Groppa and Benavides, 2008). Putrescine (Put), spermidine (Spd), and spermine (Spm) are the major PA pools commonly present in higher plants and known as active oxygen scavenging compounds being considered as mediators in protective reactions against different stresses (Kovacs et al., 2010). However, PAs can also increase ROS production through its catabolism in the apoplast by the action of Cu-containing amino oxidase (CuAO) and polyamine oxidase (PAO) activities (Smith, 1985).
We studied the effect of salt stress in the presence and the absence of MGBG, an inhibitor of S-adenosylmethionine decarboxylase (SAMDC) activity, involved in PA biosynthesis, in order to investigate the effects of both treatments on photosynthesis and oxidative metabolism providing new information about the contribution of PA metabolism to salt stress adaptation in grapevine plantlets.
Results
Salt stress applied in the culture medium of in vitro grapevine plantlets disturbed the growth rate. The application of MGBG, an inhibitor of SAMDC, resulted in further deterioration of plant growth, especially under salt stress conditions. Leaves from salt treated plantlets developed chlorotic symptoms in the leaf margins; this effect was more evident in the presence of both treatments (Fig. 1).
Salt stress produced an alteration in the fluorescence chlorophyll parameters in grapevine leaves. In this sense, a decrease in the photochemical quenching parameters [qP and Y(II)] and an increase in the non-photochemical parameters (qN and NPQ) was observed (Fig 2). The presence of the inhibitor MGBG had no important effect on qN, but it decreased NPQ values, as well as qP and Y(II) (Fig. 2).The effect of NaCl and MGBG on Fv/Fm was less pronounced when the measure was performed in the middle of the leaves. However, when Fv/Fm was recorded near the chlorotic areas (in the leaves margins) the effect of NaCl and/or MGBG was more noticeable (Fig. 2).
NaCl and MGBG treatments induced an oxidative stress as shown by the increase in lipid peroxidation level, measured as TBARS. A synergistic effect on lipid peroxidation was observed in salt-treated plantlets grown in the presence of MGBG (Fig. 3). The increase in lipid peroxidation, and therefore the damage to membrane was parallel with ROS accumulation (H2O2 and O2.-) detected by histochemical staining with DAB, or NBT, respectively (Figs. 4 and 5).
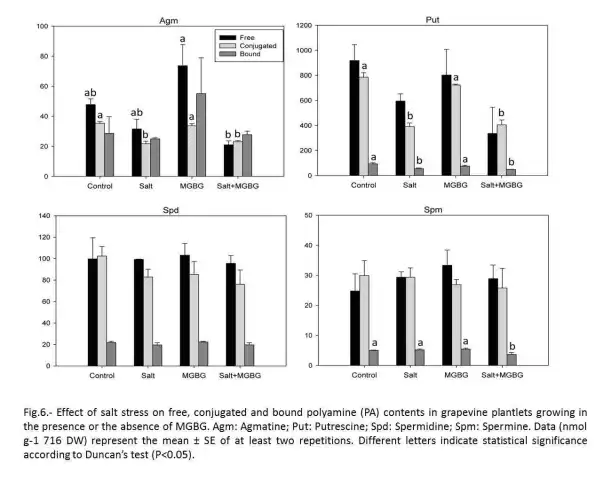
Salt treatment affected the PA contents in grapevine plantlets, especially the free and conjugate forms of agmatine (Agm) and Put. MGBG induced also a small rise in Agm content, whereas Put, Spd and Spm levels remained relatively unchanged in non-salinized plantlets (Fig. 6). The effect of salt-stress on Agm and Put was intensified in the presence of MGBG, mainly in their free forms. Surprisingly, the level of Spd remained unaffected by MGBG whatever its form, while, a 27% decrease in bound Spm was observed in the same conditions (Fig. 6).
Salt-stress induced a decrease in APX activity whereas no significant effect in MDHAR was recorded (Fig. 7). However, significant increases in SOD and POX activities were induced by NaCl (Fig. 7). The incubation of grapevine plantlets in the presence of MGBG produced no effects in APX activity, whereas significant increases in MDHAR, SOD and POX were observed, and a similar situation was recorded in the presence of both treatments (NaCl plus MGBG) (Fig. 7).
Salt-stress slightly affected the reduced ASC contents, although a strong accumulation in oxidized ascorbate (DHA) was recorded. This effect resulted in a strong decrease in the redox state of ascorbate in NaCl-treated plants (Table 1). No effect in the reduced ASC contents was observed when grapevine plantlets were incubated with MGBG. However, a significant decrease was noticed after simultaneous incubation with NaCl and MGBG (Table 1). Surprisingly, in plants treated with MGBG, in absence or presence of NaCl, no accumulation of DHA was noticed. Even a decrease in DHA in relation to control plants occurred, and accordingly, an increase in the redox state of ascorbate (Table 1). Salt-stress also produced a decrease in reduced glutathione (GSH) both in the absence and in the presence of MGBG (Table 1). In contrast, the treatment with MGBG alone had no effect in GSH contents. No significant change in oxidised glutathione (GSSG) was produced, but due to the negative effect of NaCl in GSH, a decrease in the redox state of glutathione was observed in salt-stressed grapevine plantlets (Table 1).
Results showed that MGBG treatment contribute to the deleterious effect of oxidative stress in grapevine plantlets grown in presence of NaCl, affecting different physiological and biochemical processes, including plant growth, PA levels, photosynthesis and redox state of the cells, highlighting a possible protecting role of PA homeostasis in plants subjected to salt stress.
These results suggest that maintaining polyamine biosynthesis through the enhanced SAMDC activity in grapevine leaf tissues under salt stress conditions could contribute to the enhanced ROS scavenging activity and a protection of photosynthetic apparatus from oxidative damages.
References
- Chinnusamy V, Jagendorf A, Zhu JK. (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448.
- Foyer CH, Halliwell B (1976) Presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133: 21-25.
- Groppa MD, Benavides MP. (2008) Polyamines and abiotic stress: recent advances. Amino Acids 2008; 34:35–45.
- Hernández JA, Ferrer MA, Jiménez A, Ros-Barceló A, Sevilla F. (2001) Antioxidant systems and O2.-/H2O2 production in the apoplast of Pisum sativum L. leaves: its relation with NaCl-induced necrotic lesions in minor veins. Plant Physiol 127:817-831.
- Hernández JA, Aguilar A, Portillo B, López-Gómez E, Mataix Beneyto J, García-Legaz MF. (2003) The effect of calcium on the antioxidant enzymes from salt-treated loquat and anger plants. Funct Plant Biol 30:1127-1137.
- Kovacs Z, Simon-Sarkadi L, Szücs A, Kocsy G. (2010) Different effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 38: 623–631.
- Kusano T, Yamaguchi K, Barberich T, Takahashi Y. (2007) The polyamine spermine rescues Arabidopsis from salinity and drought stresses. Plant Signal Behav 2:250-251.
- Noctor G, Foyer CH. (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49: 249-279.
- Smith TA. (1985) The di- and poly-amine oxidases of higher plants. Biochem Soc Trans 13:319-322.
For more information, please consult:
Ikbal FE, Hernández JA, Barba-Espín G, Koussa T, Aziz A, Faize M, Diaz-Vivancos P. (2014) Enhanced salt-induced antioxidative responses involve a contribution of polyamine biosynthesis in grapevine plants. J Plant Physiol. 2014, 171:779-88. doi: 10.1016/j.jplph.2014.02.006.