Diaz-Vivancos P; Bernal-Vicente A; Petri C; Cantabella D; Hernández JA
(Poster presented at the SMPR2019 congress (Valencia, 10-11 December 2019)
Abstract
Even though that salicylic acid (SA) is a key regulator of plant stress responses and other biological processes, its biosynthetic pathways have not been fully characterized. The proposed SA synthesis originates from chorismate by two distinct pathways: isochorismate and phenylalanine (Phe) ammonia-lyase (PAL) pathways. Cyanogenesis is the process related to the release of hydrogen cyanide from endogenous cyanogenic glycosides (CNglcs), and it has been linked to plant plasticity improvement. The main CNglcs in peach are prunasin and amygdalin, with mandelonitrile (MD), synthesized from Phe, controlling their turnover.
Using [13C]-labelled compounds and metabolomic analysis, we showed that MD, and hence CNglcs turnover, is involved, at least in part, in SA biosynthesis in peach plants. MD-treated peach plants displayed increased SA levels via benzoic acid (one of the SA precursors within the PAL pathway). Moreover, MD treatment modulated the H2O2 levels and had a pleiotropic effect on abscisic acid (ABA) and jasmonic acid (JA) levels (data not shown). Under the stress conditions, however, the contribution of this SA biosynthetic pathway from MD to the total SA pool does not seem to be important. Nevertheless, MD treatment not only increased SA levels, but also improved peach plants performance under the stressful conditions. In fact, MD provided partial protection against Plum pox virus (PPV) infection and stimulated the accumulation of phytotoxic ions in roots in PPV- and NaCl-stressed peach seedlings respectively.
Thus, we proposed a third pathway, alternative to the PAL pathway, for SA synthesis in peach plants, linking SA biosynthesis and cyanogenesis (Fig. 1). This proposed pathway seems to be functional under stress conditions, although the fact that CNglcs may be operating more broadly than by influencing SA pathways and signaling con not be ruled out.
Material and Methods
Plant material
The assays were performed on GF305 peach (Prunus persica L.) plants, under both greenhouse and in vitro conditions. For ex vitro assays, after submitting the GF305 peach seedlings to an artificial rest period in a cold chamber to ensure uniformity and fast growth, seedlings were grown in 2-L pots in an insect-proof greenhouse and distributed into three batches (control and MD- and Phe-treated) of 15 plants each. Plants were irrigated twice per week with water (control) and 1 mM MD or 1 mM Phe for 7 weeks. None of the treatments affected plant growth and development. For in vitro assays [13C]-labeled compounds were used, and 200 mM MD- or Phe-alpha[13C] (Campro Scientific GmbH, Germany) was added to the micropropagation medium during two subcultures. This concentration was selected after carrying out a preliminary assay using concentrations of both compounds ranging from 50 to 1000 mM; 200 mM was the highest concentration that did not show any deleterious effect on the development of micropropagated peach shoots.
Metabolomic analysis
The levels of Phe, MD, amygdalin, BA and SA were determined in in vitro micropropagated shoots at the Metabolomics Platform at CEBAS-CSIC (Murcia, Spain).
SA levels in peach seedlings
The SA levels in leaves of GF305 seedlings treated with MD or Phe were determined using a UHPLC-mass spectrometer (Q-Exactive, ThermoFisher Scientific) at the Plant Hormone Quantification Platform at IBMCP (Valencia, Spain).
Extraction and enzymatic assays
The activities of ascorbate peroxidase (APX), peroxidase (POX), catalase (CAT) and superoxide dismutase (SOD) in in vitro shoots and ex vitro leaf samples were assayed as described in Diaz-Vivancos et al. (2017) and Bernal-Vicente et al. (2018; 2019).
Results
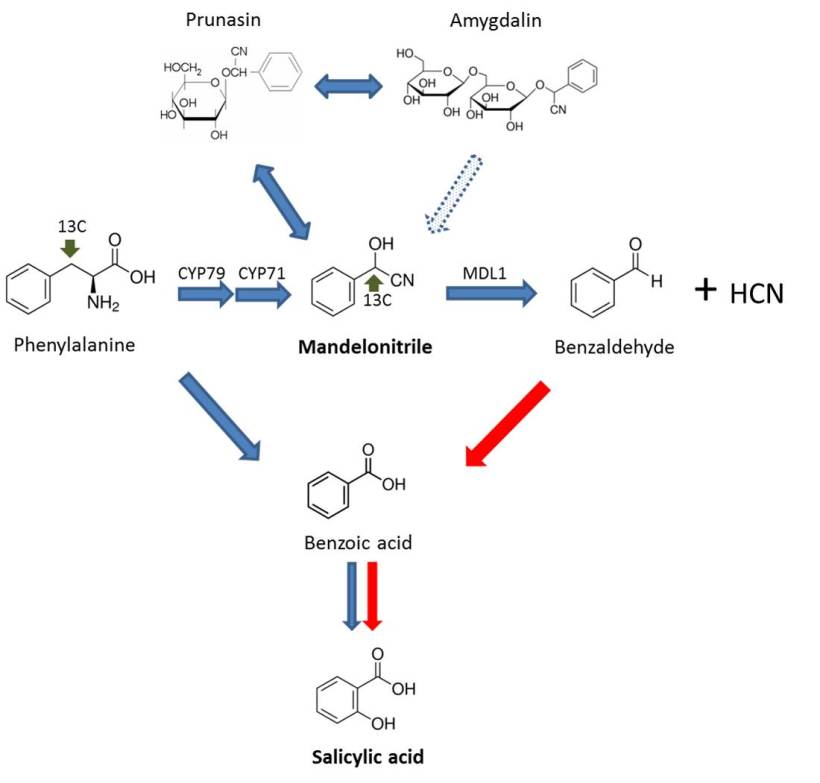
Fig. 1. Proposed salicylic acid (SA) biosynthetic pathway in peach plants. Blue arrows indicate the SA biosynthesis in plants described previously. The dotted arrow indicates a putative pathway. Red arrows show the new pathway suggested for peach plants. CYP79 and CYP71, Cyt P450 monooxygenases; MDL1, mandelonitrile lyase.
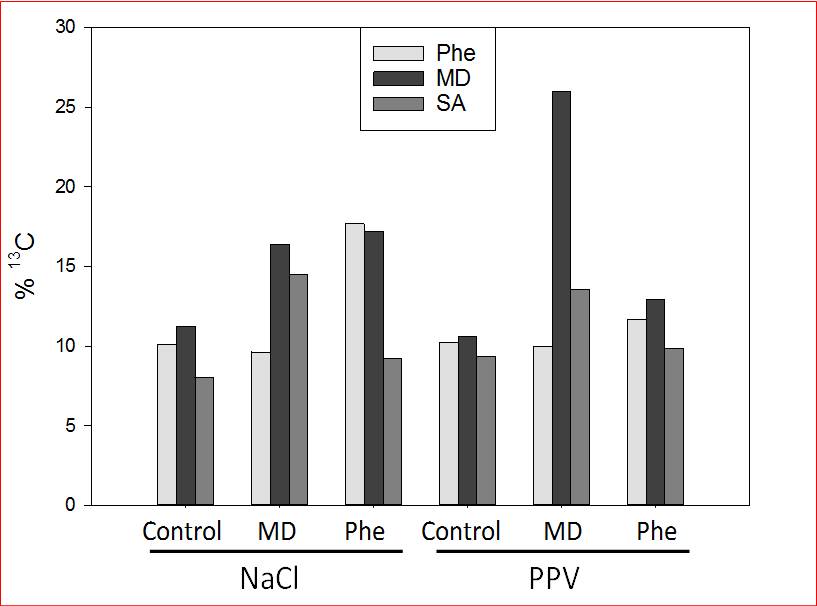
Fig. 2. Percentage (of total amount detected) of [13C]- phenylalanine (Phe), mandelonitrile (MD) and salicylic acid (SA) in non-stressed, NaCl-stressed and Plum pox virus (PPV)-infected peach shoots micropropagated in the presence or absence of [13C]MD or [13C]Phe. Ions with an additional 1.0035 accurate mass and confirmation by isotopic distribution and spacing were defined as ions marked with 13C. Data represent the mean of at least 20 repetitions of each treatment.
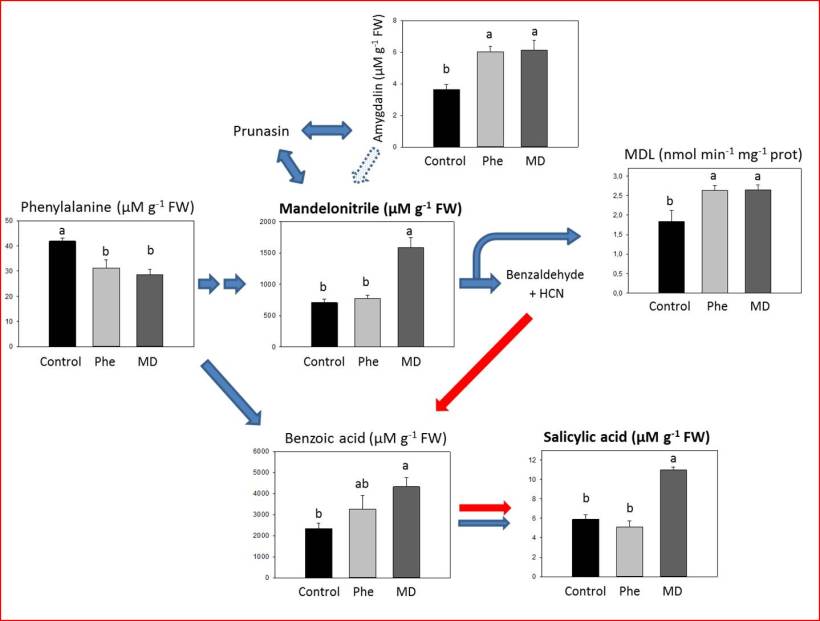
Fig. 3 Total levels (mMg–1 FW) of amygdalin, benzoic acid, mandelonitrile, phenylalanine and salicylic acid, and mandelonitrile lyase (MDL) enzymatic activity in micropropagated peach shoots in the presence or absence of [13C]MD or [13C]Phe. Data represent the mean ± SE of at least 12 repetitions of each treatment. In each graph different letters above the columns indicate significant differences according to Duncan’s test (P< 0.05).
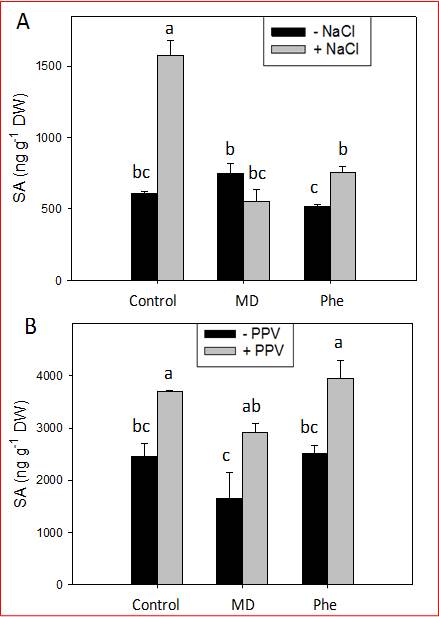
Fig. 4. Total SA level (ng g–1 DW) in the leaves of peach seedlings grown in the presence or absence of MD or Phe submitted to 34 mM NaCl (A) or PPV infection (B). Data represent the mean ± SE of at least five repetitions of each treatment. Different letters indicate significant differences according to Duncan’s test (P≤0.05).
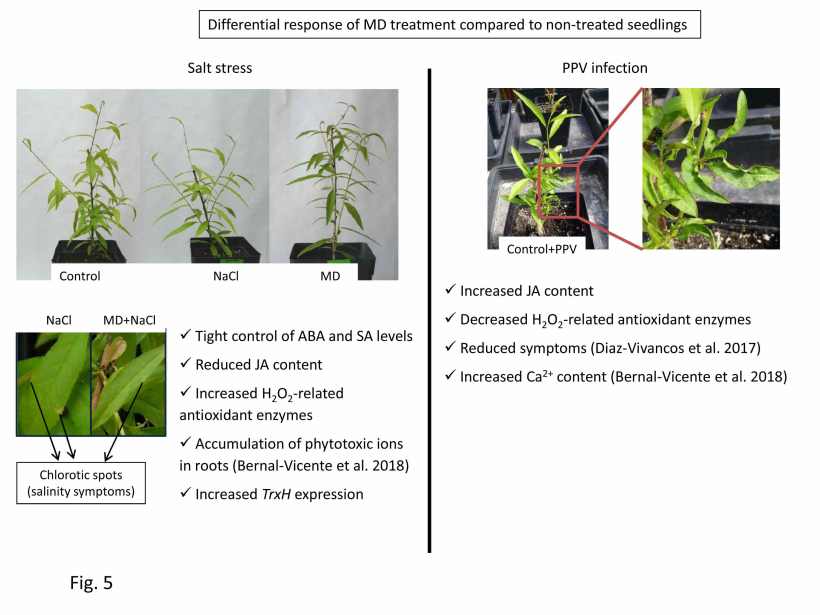
Fig. 5. Summary of the differential response of MD treatment in salt-stressed and PPV-infected peach seedlings. Data from Diaz-Vivancos et al. (2017) and Bernal-Vicente et al. (2018; 2019).
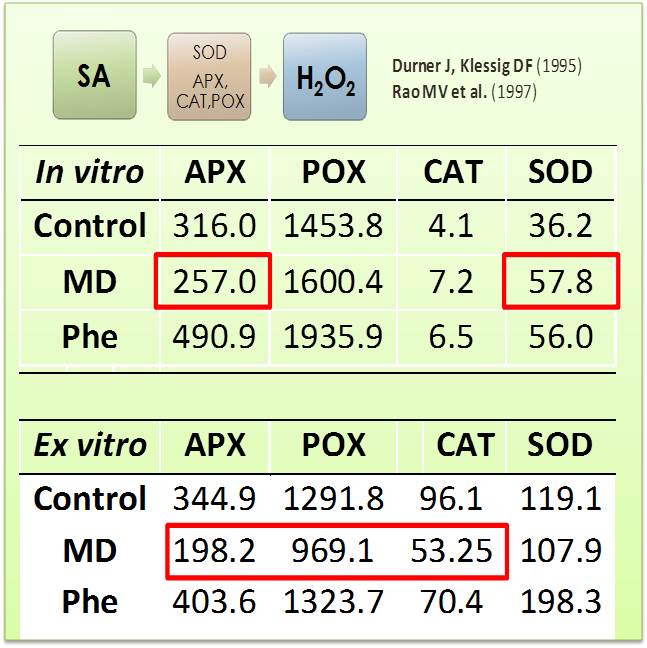
Table 1.- Effect of MD and Phe on H2O2-scavenging activities (APX, POX, CAT) and SOD (H2O2-producing) activity in GF305 micropropagated shoots and seedlings. APX is expressed as nmol min-1 mg-1 protein. POX and CAT are expressed as µmol min-1 mg-1 protein. SOD as U mg-1 protein. Data represent the mean ± SE of at least four repetitions. It has been previously described that SA led to H2O2 accumulation (Durner & Klessig 1995; Rao et al. 1997).
Conclusions
We provide strong evidences showing that CNglcs turnover is involved, at least in part, in SA biosynthesis in peach plants under control and stress conditions.
MD seems to act as a hub controlling the CNglcs turnover and the SA and amygdalin biosynthesis. MD-treated peach plants displayed increased SA levels via benzoic acid (one of the SA precursors within the PAL pathway).
We have also found evidence that this new SA biosynthetic pathway also works also under stress conditions. MD treatment improved the plant performance of peach plants under salinity or PPV-infection conditions. However, the contribution of this pathway to the SA pool does not seem to be relevant under these stress conditions.
MD also modulated the content in other stress related hormones as well as the antioxidant defenses, that could activate different redox-related signaling pathways.
Our data agrees with the previously reported role for CNglcs in oxidative stress tolerance (Gleadow & Møller 2014; Gleadow et al. 2016).
References
- Diaz-Vivancos et al (2017) Plant & Cell Physiology 58(12): 2057–2066
- Durner J and Klessig DF (1995) Proc. Natl. Acad. Sci. U SA 92(24): 11312-11316
- Bernal-Vicente et al (2018) Plant Biology 20(6):986-994
- Bernal-Vicente et al (2019) Plant Biology DOI: 10.1111/plb.13066
- Gleadow Møller (2014) Annual Review of Plant Biology 65:155–185
- Gleadow et al (2016) Journal of Experimental Botany 6:403-5413
- Rao et al (1997) Plant Physiology 115:137–149.
This work was supported by the Spanish Ministry of Economy and Competitiveness (projects AGL2014-52563-R; RTA2017-00011-C03-02).
